What is compliance automation?
Compliance automation, also known as automated compliance, is the practice of using technology -- such as applications with AI features -- to perform and simplify compliance procedures. Whether it's adhering to the Health Insurance Portability and Accountability Act (HIPAA), General Data Protection Regulation, California Consumer Privacy Act or another regulatory standard, most organizations must follow set rules that govern how they should manage business operations, like data, security and risk.
Compliance automation software is used to replace tedious manual processes, streamlining regulatory workflows. The practice also helps to reduce errors caused by human-based compliance and alert fatigue. When implemented well, compliance automation can increase operational productivity and lead to a state of continuous compliance.
How does compliance automation work?
Automated compliance software provides organizations with compliance workflow capabilities, such as self-assessment, corrective action planning, and controls analysis and testing. Automating these processes can replace manual spot checks.
Many compliance monitoring tools are set up with the organization's security policies. To detect violations, regulations related to a company's industry, configurations, accounts, inventories and security measures are copied into the compliance automation software. The organization can change or update this knowledge base of compliance regulations and security standards at any time.
In some cases, the compliance software also comes preloaded with common regulatory standards, enabling teams to map internal policies directly to those frameworks.
Compliance automation software relies on effectively integrating with an organization's in-place hardware and software systems. The tools also increasingly use AI, machine learning and data analytics to detect anomalies and continuously monitor for potential compliance risks.
As an example, healthcare providers who handle patient records are subject to HIPAA regulations. These providers can use compliance automation software to continuously monitor business processes that include electronic health record systems, access controls and encryption settings. The software can automatically compare the organization's current security configurations against HIPAA's standards. If protected health information is accessed without proper authorization, the system can flag the violation and initiate a predefined response, which helps ensure a level of continuous compliance.
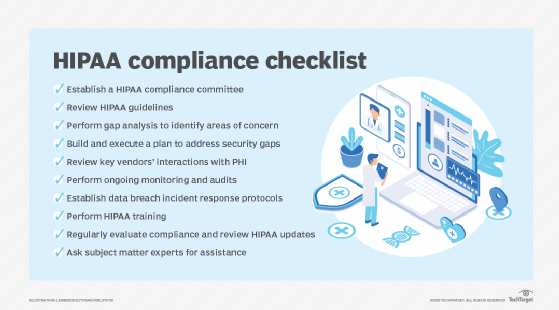
Benefits of compliance automation
Automated compliance is beneficial for organizations that process sensitive information or that must adhere to stringent rules and standards for protecting customer data, such as hospitals and banks. In light of continuously evolving requirements, automating regulatory compliance processes can improve productivity and accuracy for internal auditors and senior management in these sectors. Automating compliance processes has several other benefits, including the following:
- Automated compliance procedures are more time-saving and cost-effective than manual controls.
- Personnel can access and check compliance status and audit information on a single dashboard.
- Real-time data helps leaders make more informed risk management decisions.
- Automation makes it easier to implement uniform compliance policies across IT environments, including physical servers, private clouds, public clouds and containers.
- Automated compliance software can continuously verify compliance requirements, manage third-party risks and catch potential weaknesses.
- Automation can minimize inadequacy and inaccuracy in compliance workflows and reporting, reducing the likelihood of fines and other penalties for breaches.
How to implement compliance automation
Compliance automation looks different for each organization that implements it, as different factors change depending on what regulations need to be complied with. Some general steps to follow when implementing compliance automation, however, can include the following:
- Define compliance requirements. This step should include identifying all regulations, standards and internal policies that the organization must follow.
- Assess current compliance processes, and plan automation strategies. Existing workflows and policies should be assessed to identify gaps, inefficiencies and areas that would benefit from automation.
- Select compliance automation tools. Select tools that fit the organization's needs and can be integrated with existing systems.
- Develop automation workflows. Detailed records should be kept and maintained regarding automated tasks, like evidence collection, alerts and response measures.
- Set up the compliance tool. The chosen tool is set up and integrated with existing IT, human resources, security or operational tools.
- Continuously update and monitor performance. Automation workflows should be regularly reviewed and updated as external and internal regulations change.
Assessing compliance automation tools
There are multiple compliance automation tools an organization can choose from. The following is a sampling of different available tools:
- ActiveState.
- CloudBees.
- Drata.
- Fortinet.
- Hyperproof.
- Sprinto.
- Vanta.
Organizations should consider their compliance needs when choosing a compliance automation platform, as different platforms might prioritize different compliance standards. Likewise, it is equally important to know what other tools the platform can integrate with.
Before investing and implementing a platform, organizations should also evaluate the features and capabilities of each of their considered platforms, the level of support the vendor offers and the total cost of ownership, while also requesting trials.
What's the future of compliance automation?
Some of the bigger changes to compliance automation platforms in the last few years -- and likely to come -- have been the increased use of AI, machine learning and cloud-based systems that can manage regulatory compliance in real time. The locations of workers have changed since the COVID-19 pandemic as well, with many businesses having since integrated remote and hybrid work, meaning more must be done to ensure compliance with different regulatory standards.
There might also be more of a movement toward continuous compliance instead of quarterly or annual checks, which fits in well with continuous integration/continuous delivery and cloud-based workflows. The use of AI and machine learning tools enables organizations to monitor regulatory changes continuously, while also automating repetitive tasks with greater accuracy.
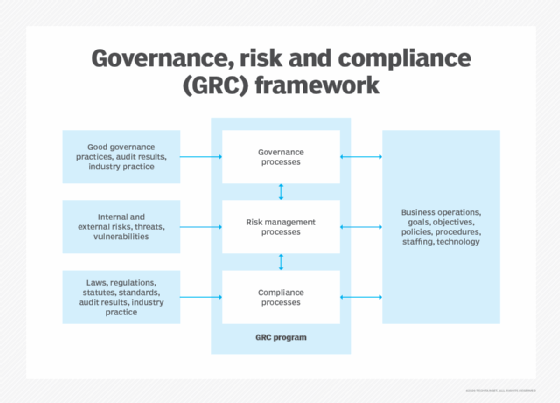
Implementing governance, risk and compliance systems can be a challenge. Learn more about the steps to successfully automate these systems.






